Tragic, preventable incidents continue to occur during K-12 science demonstrations involving flammable materials.
Despite communication efforts and safety alerts sent out by the American Chemical Society, the US Chemical Safety Board, National Fire Protection Association, and National Science Teachers Association, tragic and preventable incidents are occurring during science demonstrations involving flammable materials. Most recently, another incident “Four students injured in science experiment gone wrong at Bronx school” occurred on November 22, 2017 at an high school academy in New York City.
 |
 |
| A dangerous approach: Uncontained flammable liquids burning in dishes with excess fuel nearby and inadequate ventilation.. |
Much safer: Soaking wooden splints in salts and viewing the colors produced by burning the splint. |
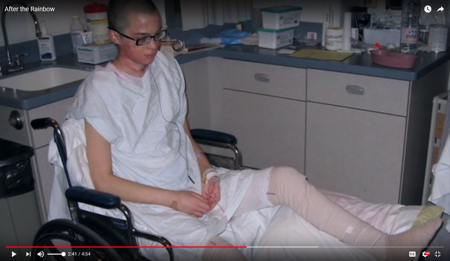
Since 2006, over 90 children have been burned in these incidents, some as young as 3 yrs old. For links to articles and dates of these incidents are available upon request.
 |
 |
| Calais Weber was burned on over 40% of her body in 2006 at Western Reserve Academy in Ohio. (Source US Chemial Safety Board) |
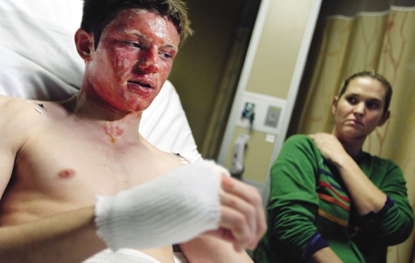
Dane Neuberger, a ninth grader in Minnesota who was one of four students burned in a science demonstration involving methanol. “My face was actually on fire,” he told local media.
(Photograph: Richard Tsong Taatarii/Minneapolis Star Tribune) |
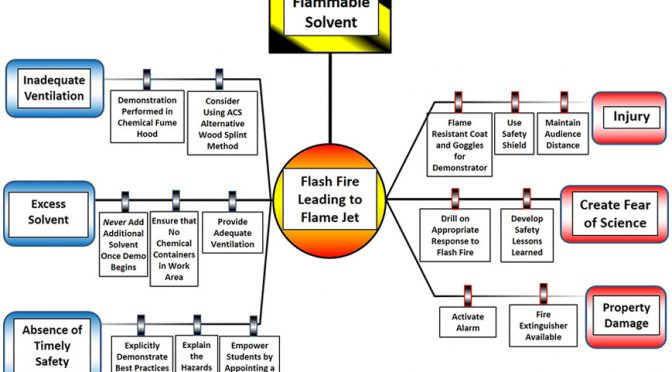
Why Does This Keep Happening?
In many of the cases where injuries have occurred, the demonstrator has tried to sustain the flame by adding additional fuel to a hot evaporating dish or a dish where the methanol flame has not gone out, but is not visible. When a 1- or 4- liter container is used by the demonstrator, a phenomenon known as “flame-jetting” can occur. A NFPA Sep/Oct 2017 article explains flame jetting with images from the Bureau of Alcohol, Tobacco, Firearms and Explosives (ATF) testing at the Fire Research Lab (FRL).
The ATF FRL testing found that flame jetting:
- Occurred in all 49 tests using ethyl alcohol in various types and sizes of containers, including glass and plastic containers in one-liter and one-gallon sizes, the sizes used in the rainbow demonstration incidents.
- Flame jets in excess of 15 feet occurred during testing. This is consistent with witness statements and fire damage in the classrooms.
- The entire jetting event lasted less than one second, with no observable warning signs prior to the phenomenon. When jetting did occur, there was no evidence of thermal or pressure damage to the container.
- Some flammable liquids such as fresh gasoline are able to release flammable vapors readily enough that the headspace never drops below the upper flammable limit and therefore does not support flame propagation within the container. Weathered gasoline, by comparison, is slower to release vapors and can support flame propagation inside the container, leading to flame jetting.
Similar incidents have occurred with portable plastic gas cans that lack flame arresters, resulting in over 11 deaths and 1200 emergency room visits.
Flame jetting is most likely to happen when:
- The temperature of the fuel is room temperature or lower, so vapors collect in the container, instead of evaporating out of it and only move when poured out.
- The pouring angle allows vapors to travel out and flash back inside the container. when the container is upright, the head space above the liquid is too fuel-rich and above the upper flammability limit, meaning that combustion is not supported within the container. As the container is tilted and vapors begin to pour from the open mouth, however, air is entrained into the head space and the fuel-rich mixture eventually falls within the flammable limits; if an ignition source is present and combustion occurs, the flame propagation condition inside the container can lead to flame jetting.
- There is little fuel left in the can, making it easier for flashback to get through the bottle opening.

Figure from http://www.notyourturntoburn.com/flame-arresters/
What Can Be Done?
Flame arresters have been shown in testing to prevent flame jetting from portable flammable liquid containers that would otherwise produce jets in certain conditions.
The organization Not your turn to burn, which has been organized by mothers of burn victims, has extensive information about the mechanisms behind flame jetting and advocates for flame arresters in The Portable Fuel Container Safety Act of 2017, currently in committee, would require flame arresters on portable flammable liquid containers. Fire prevention advocates say that adding a flame arrester to the opening of a container costs when the container is manufactured will cost less than 50 cents on most portable fuel containers.
This phenomenon is not unknown to the ethanol industry and some manufacturers routinely install flash arresters such the grates on alcoholic beverages such as this Bacardi 151, as manufacturers are aware of flaming drinks.

Flame arresters are required by OSHA for workplace use but not by Consumer Product Safety Commission (CPSC); many feel that making a packaging option on bottles of common alcohols similar to those on alcoholic beverages could prevent more household tragedies.
Action Items:
- Please share the following resources and reminders with your local schools districts and teachers to try to spread the word regarding the dangers of these types of experiments and safer alternatives.
- Encourage your congressional representatives to support H.R.919: Portable Fuel Container Safety Act of 2017. It has 31 co-sponsors and is currently in the House Energy and Commerce committee. Letter templates available at http://www.notyourturntoburn.com/letters-articles/
- Share with everyone to NEVER add flammable liquids to an open flame. This can lead to FLAME jetting, Use containers with flash arresters.
- Petition container manufacturers to sell flame arrester liners compatible with common caps of flammable liquid containers.
- Petition chemical manufacturers to add flame arresters to bottles of flammable liquids, similar to the Safety-pour technology provided on bottles available from Lumina Products (https://youtu.be/AS5WDA7mAvw)
Resources
National Science Teachers Association resources
National Fire Protection Association
Template for writing local school officials on this issue
Superintendent
School District
Address
Dear Dr. Superintendent:
As a parent of a current student/ scientist/ concerned community member/ member of the American Chemical Society’s (ACS) Division of Chemical Health and Safety- I’m writing to you today to ensure that you were aware of recent accidents involving unsafe scientific demonstrations and that your district has policies in place for ensuring the safety of scientific demonstrations in your schools. In addition, I would like to make you aware of information that ACS produced specifically related to the safe handling of chemicals for educational purposes.
The driving force behind my correspondence today was the recent report of several students being injured during a “chemistry experiment gone wrong”. Details on this specific event are still slim, but unfortunately seem to be consistent with several recent events in which students have been injured during demonstrations involving flammable materials. One such experiment, the ‘rainbow demonstration’ has been the cause of numerous accidents resulting in severe student and teacher injuries. In spite of many news reports and multi-million-dollar settlements- these accidents continue to happen.
So we are reaching out directly to our local school districts and teachers to try to spread the word regarding the dangers of these types of experiments and safer alternatives. The ACS, National Fire Protection Agency, and the US Chemical Safety Board have all created documents highlighting these dangers and guidelines for providing safe and educational demonstration. I’ve included several links to these (free) resources below and would be glad to discuss this more with you if desired.
Sincerely,
Your Name,
Address and Phone